

Every Safety Data Sheet (SDS) acts as the frontline reference for chemical hazards. When it’s out of date, workers lose access to crucial information on exposure limits, PPE requirements, and emergency actions.
Accurate, timely revisions therefore safeguard employee health, streamline audits, and prevent fines. Particularly for industries with frequent formulation changes or ever-changing toxicology data.
| Jurisdiction | Update Deadline | Relevant Rule / Standard |
|---|---|---|
| OSHA – Hazard Communication Standard | Within 3 months of becoming aware of new or significant hazard information that changes classification or protective measures | 29 CFR 1910.1200 (g)(5) |
| EPA – Emergency Planning and Community Right-to-Know Act (EPCRA) | Submit the revised SDS (and, if applicable, Tier II reports) to state emergency response commissions (SERCs), local emergency planning committees (LEPCs), and fire departments once the OSHA trigger applies | 40 CFR 370 (Subpart C) |
Key Triggers That Demand an Update
SDS updates are not tied to a calendar but are required whenever certain events occur. The most common triggers include:
- Discovery of New Hazards
This could include new toxicology data, reclassification of health effects, or changes in permissible exposure limits. - Changes in Formulation or Supplier
Even small shifts in ingredients or manufacturing sources can alter a product's hazard classification. - Regulatory Actions
New GHS classifications, restrictions, or authorizations may affect how a chemical must be communicated. - Operational Feedback
Safety incidents or internal investigations can reveal gaps or inaccuracies in existing SDS content. - Advancements in Protective Measures
If better PPE or engineering controls are introduced, the SDS must reflect them.
When any trigger surfaces, OSHA gives manufacturers and importers three months to reissue the SDS, and distributors must pass it along with the next shipment.
Recommended Review Cycles & Best Practices
Although OSHA does not enforce a fixed review cycle, proactive organizations follow structured intervals to stay ahead:
| Review type | When / trigger | What to do | Best-practice tip | Outcome |
|---|---|---|---|---|
| Quarterly Reviews | Every quarter | Monitor supplier and agency bulletins for changes that affect your SDS in use. Log any changes and flag impacted documents. | Subscribe to vendor bulletins and regulatory updates. Maintain a simple change log. | Catch changes early; reduce compliance gaps. |
| Annual Audits | Once per year | Compare your SDS files with the latest manufacturer versions. Revalidate any document older than two years. Update binders and links. | Use a master inventory and version control checklist. | Ensure accuracy and legal compliance. |
| Post-Incident Checks | After any spill, exposure, or near-miss | Recheck the SDS for clear, current emergency response steps. Update training, signage, and procedures as needed. | Conduct a short debrief with EHS officers and supervisors. Record corrective actions. | Close gaps and prevent repeat events. |
These review habits ensure you don’t rely solely on external notifications to keep your safety documentation accurate.
Building an Effective SDS Update Workflow
Maintaining a robust SDS update process includes:
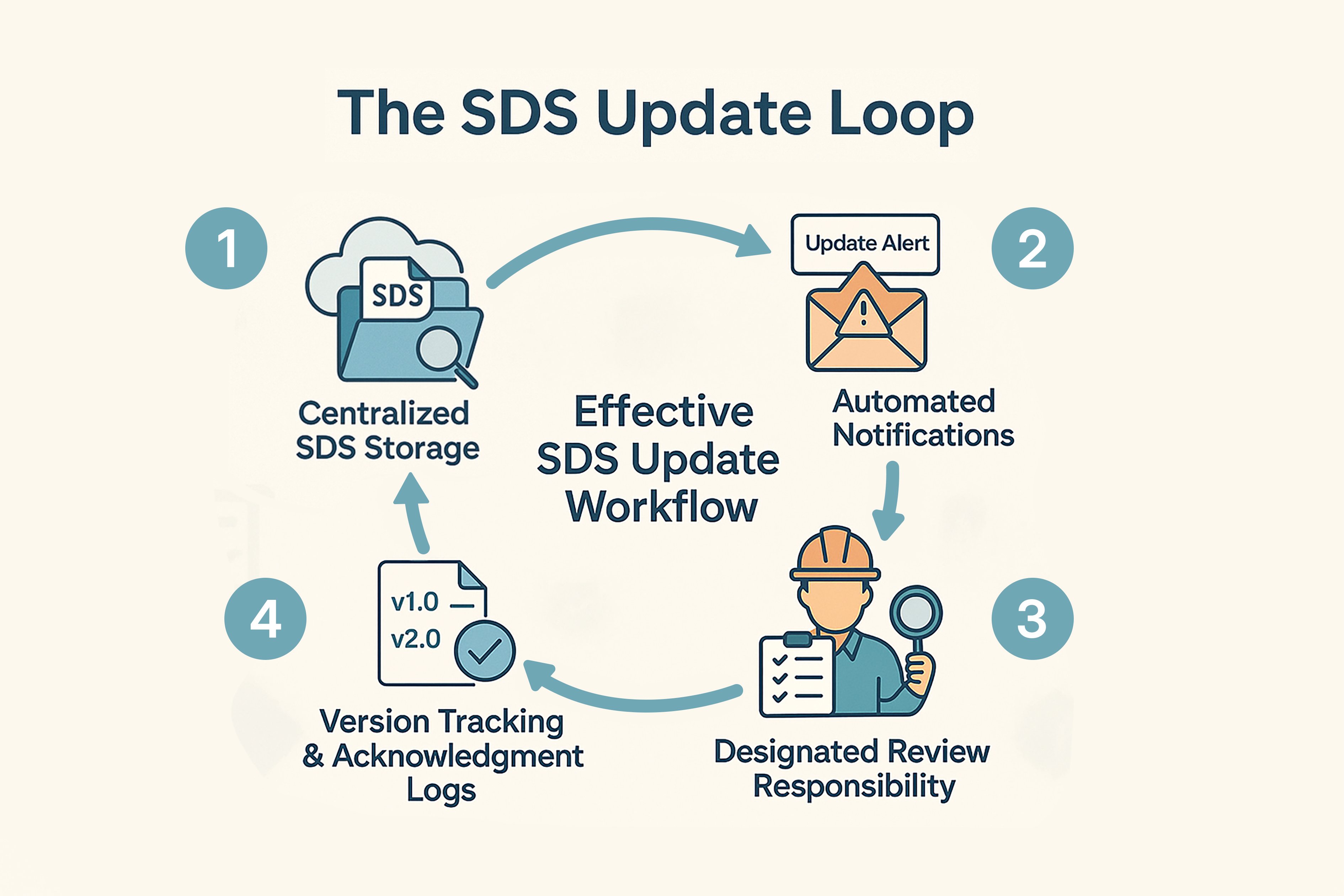
1. Keep all SDS in a searchable digital library (cloud or secure local system) with role-based access.
2. Enable alert systems for supplier updates and regulatory bulletins.
3. Assign a dedicated EHS lead or SDS coordinator to track changes, verify relevance, and manage distribution.
4. Maintain audit trails showing when employees accessed revised SDS and confirmed their review.
Staying Ahead of Future Compliance Changes
OSHA’s 2024 updates aligned further with GHS Revision 7, refining how classifications and hazard statements are defined. These ongoing changes signal that SDS management must evolve too. Staying compliant requires watching regulatory trends and preparing early instead of waiting for triggers. Subscribe to OSHA and EPA mailing lists, follow industry associations, and make sure your team reviews proposed rule changes before they take effect.
Build internal habits like prompting SDS review after supplier changes and clear documentation of staff acknowledgments.This helps your organization stay ahead of new requirements and maintain hazard communication that meets both legal and safety expectations.
FAQs
- How often must an SDS be updated?
Whenever new, significant hazard information becomes available. OSHA requires updates within three months of discovery. - Is there a fixed expiration date for SDS?
No. OSHA does not set a time-based expiration, but regular reviews are recommended to ensure relevance and accuracy. - Who is responsible for issuing updated SDSs?
Manufacturers and importers must create them, while distributors and employers are responsible for ensuring accessibility and communication. - Do SDS need updates for small formulation changes?
Yes, if the change impacts the product’s classification or safety guidance, the SDS must be revised. - Are electronic SDS accepted under OSHA?
Yes. Electronic formats are acceptable as long as employees can access them immediately during their shift, and there is a backup method.