

Every Safety Data Sheet (SDS) serves as a frontline document for chemical hazard communication. When it’s outdated, workers may lack essential information on exposure limits, appropriate PPE, or emergency response actions.
Ensuring that SDSs are regularly reviewed and updated helps protect health, support compliance inspections, and maintain safety. This is particularly true for industries where chemical compositions change often or regulatory classifications are revised.
Regulatory Requirements at a Glance
| Jurisdiction | Update Deadline | Relevant Rule / Standard |
|---|---|---|
| UK REACH | Without undue delay upon becoming aware of new information that affects hazard classification or risk management | Article 31 of UK REACH (retained Regulation (EC) No 1907/2006) |
| CLP Regulation | When new data on hazards or classification becomes available | Regulation (EC) No 1272/2008, as retained in UK law |
| COSHH | Employers must ensure SDS used in risk assessments are current and reflect known hazards | Regulation 12, Control of Substances Hazardous to Health Regulations 2002 |
Key Triggers That Demand an Update
SDS updates are event-driven, not time-bound. Common reasons to revise include:
- New Hazard Information
Examples include updated toxicological data or changes to health hazard classifications. - Changes in Composition
A shift in supplier, ingredients, or formulation that alters hazard properties will require an SDS update. - Regulatory Revisions
Updates to GHS classification criteria, substance restrictions, or labelling obligations prompt a review. - Workplace Feedback
Internal incident reports, risk assessments, or PPE evaluations may reveal inconsistencies in existing SDS data. - Better Risk Controls
If a new control measure, such as improved PPE, is introduced, the SDS should reflect this advancement.
Suppliers must distribute the revised SDS as soon as new data becomes available, and employers must ensure it's accessible and incorporated into their safety procedures.
Recommended Review Cycles & Best Practices
Though not mandated by a time-based rule, routine reviews are considered best practice:
| Review type | When / trigger | What to do | Why it matters |
|---|---|---|---|
| Quarterly Monitoring | Every quarter | Track industry bulletins and chemical agency updates; note any SDS-impacting changes. | Catches regulatory or supplier updates early. |
| Annual File Audits | Once per year | Review the SDS archive; if any sheet is >2 years old, cross-check against the latest manufacturer version. | Keeps files current and defensible in audits. |
| Incident-Based Checks | After any spill, exposure, or near-miss | Reassess the SDS to confirm emergency response guidance is clear and up to date; update training/procedures if needed. | Ensures readiness and prevents repeat events. |
These practices help ensure SDS accuracy without waiting for supplier notifications alone.
Building an Effective SDS Update Workflow
A structured system for SDS reviews supports ongoing compliance:
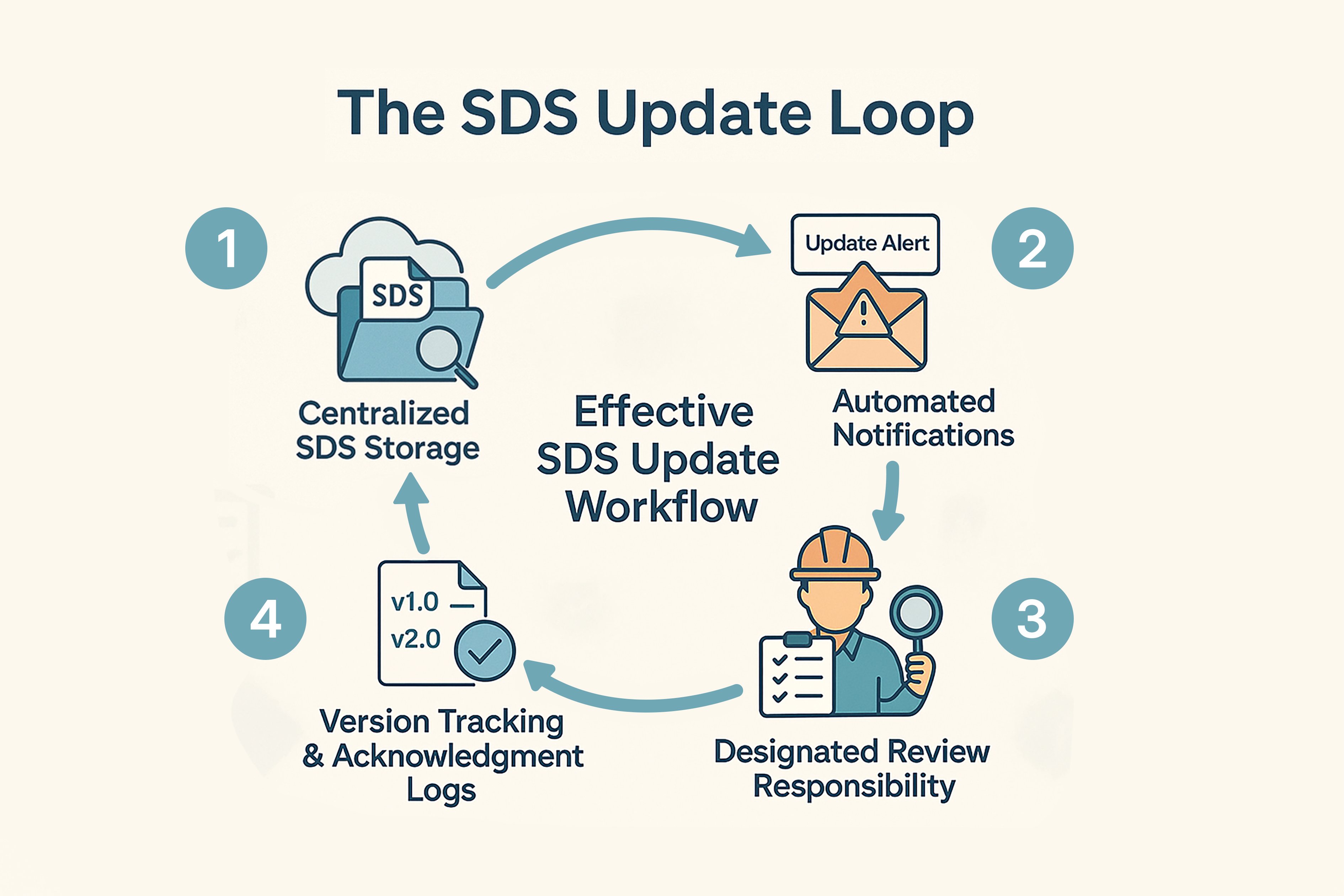
- Maintain SDS in a digital platform accessible to all relevant staff.
- Use subscription tools or supplier systems to receive updates.
- Appoint a responsible person or team to evaluate updates, validate accuracy, and redistribute revised versions.
- Keep records of when employees accessed new SDS files, including any confirmations of understanding.
Staying Ahead of Future Compliance Changes
Chemical safety regulations continue to evolve. The UK's adoption of GHS-based systems, and the ongoing updates to CLP classifications and REACH authorisation lists, mean that SDS relevance is not static.
Staying compliant requires being prepared for regulatory changes. By subscribing to updates from the Health and Safety Executive (HSE) and chemical safety networks, organisations can proactively identify shifts in hazard classifications, restricted substances, and labelling guidance.
Internally, this foresight should be matched with procedures that document staff awareness and make revised SDS available before noncompliance becomes a risk.
FAQs
- How often should an SDS be updated?
There’s no fixed interval. SDS must be updated promptly whenever new hazard data or regulatory changes affect safety information. - Is there an expiry date for SDS?
No, but if an SDS is more than two to three years old, it should be reviewed to ensure it still reflects current substance and classification data. - Who is responsible for keeping SDS up to date?
Suppliers must revise SDS when necessary. Employers are responsible for maintaining accessible, accurate versions for all chemicals used on site. - Do minor changes in composition require an update?
Yes, if the change alters hazard classification, risk management advice, or required PPE. - Can SDS be stored digitally?
Yes. Digital formats are acceptable as long as employees can access them without delay and alternative access methods are available in case of IT failures.