

Every Safety Data Sheet (SDS) is a cornerstone of chemical hazard communication in Canadian workplaces. An outdated SDS can mean employees are missing critical information, such as revised PPE guidance, updated emergency procedures, or new classifications.
Keeping SDS current not only protects workers but ensures compliance with national legislation under WHMIS 2015.
Regulatory Requirements at a Glance
| Jurisdiction | Update Deadline | Relevant Rule / Standard |
|---|---|---|
| HPR (Hazardous Products Regulations) | Within 90 days of becoming aware of significant new data about a hazardous product | Section 5.12 of the HPR under the Hazardous Products Act |
| WHMIS 2015 | SDS must reflect accurate, up-to-date hazard information before a product is sold or imported | WHMIS aligned with GHS, regulated by Health Canada |
| Provincial/Territorial OHS Acts | Employers must ensure SDS are available, legible, and reflect the current version received from suppliers | Occupational Health and Safety laws vary by province, but align with WHMIS standards |
Key Triggers That Demand an Update
SDS Updates are required when:
- New Data Emerges
Toxicology updates, reclassification of hazards, or new scientific findings. - Changes in Composition or Supplier
Any formulation or supplier change that impacts the product’s hazard profile. - Legislative or Regulatory Revisions
Updates to WHMIS or HPR classifications or labelling formats. - Workplace Use Review
Internal feedback that reveals discrepancies or information gaps in SDS content. - Improved Control Measures
Advancements in ventilation, PPE, or risk control that alter the handling recommendations.
Once significant new data becomes known, suppliers have 90 days to update the SDS and 180 days to update the product label. Employers must ensure employees have access to the revised SDS immediately upon receipt.
Recommended Review Cycles & Best Practices
To ensure ongoing compliance beyond legislative requirements:
| Review type | Frequency / trigger | Primary action | Documentation tip | Why it matters |
|---|---|---|---|---|
| Quarterly Monitoring | Every quarter (calendar-based) | Track supplier bulletins and Health Canada updates; note SDS impacts. | Keep a simple change log tied to product/CAS. | Catches changes early and reduces compliance risk. |
| Annual SDS Audit | Once per year | Inventory SDS; flag any 2–3+ years old and request supplier confirmation or updates. | Maintain a master SDS register with version dates. | Keeps the archive current and audit-ready. |
| Post-Incident Review | After any spill, exposure, or near-miss | Recheck relevant SDS for accurate emergency guidance; update training and SOPs if needed. | Record findings and corrective actions in the EHS log. | Strengthens response and prevents repeat events. |
While not legally required on a fixed schedule, these checks reinforce control and confidence in your chemical safety system.
Building an Effective SDS Update Workflow
Effective SDS management involves:
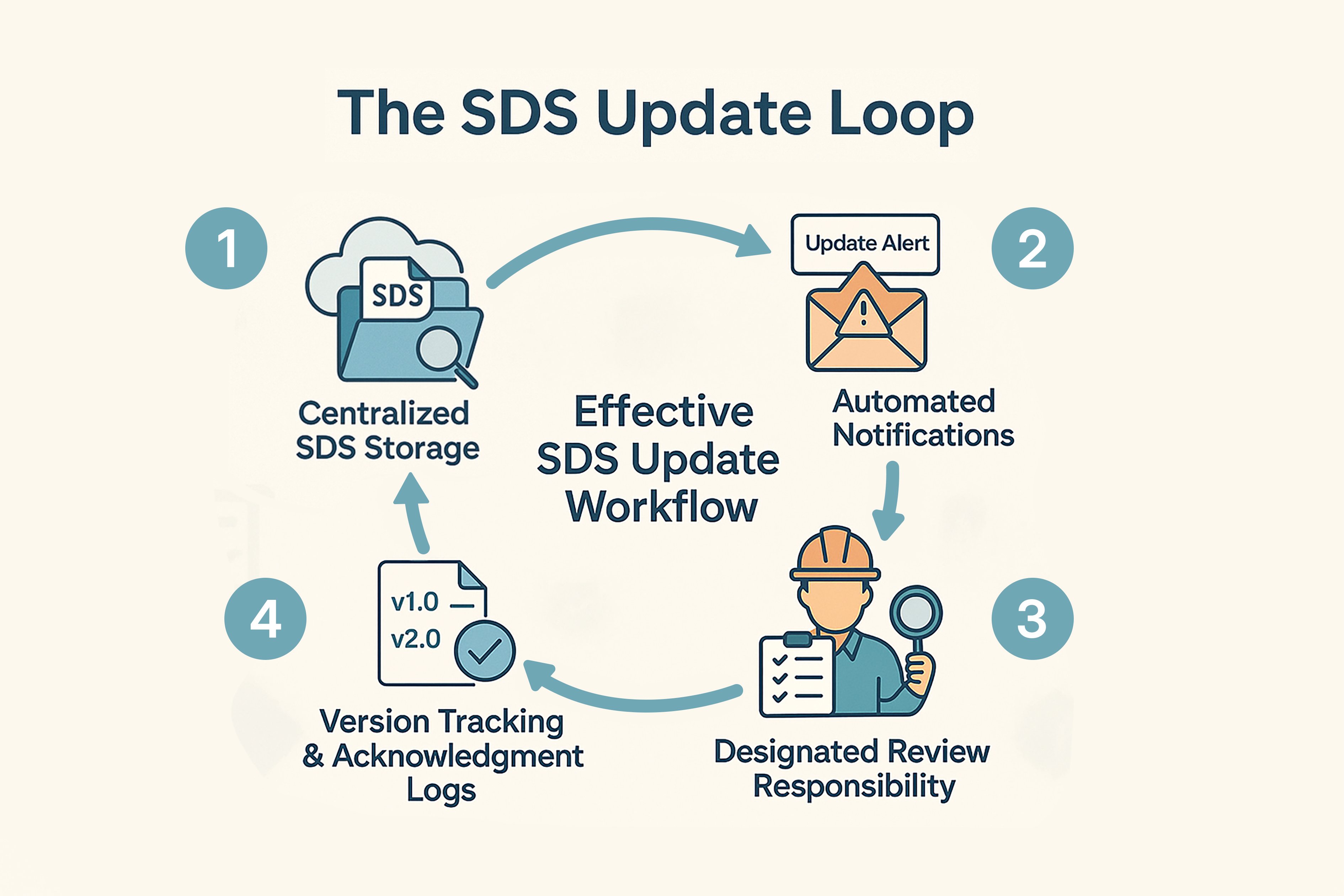
1. Centralised and accessible by staff in every relevant work area.
2. Subscriptions or email notifications from suppliers or regulatory bodies.
3. A competent person or team should own SDS review and communication processes.
4. Use version logs and read confirmations to show compliance during inspections.
Staying Ahead of Future Compliance Changes
WHMIS is evolving, with future updates expected to align more closely with the latest GHS revisions. By staying subscribed to Health Canada bulletins and chemical safety associations, employers can anticipate shifts in classification systems, hazard categories, and SDS structure.
Internally, maintaining procedures for quick SDS distribution and version tracking will ensure readiness as regulatory expectations evolve.
FAQs
- How often should SDS be updated in Canada?
When suppliers receive significant new data, SDS must be updated within 90 days. - Do SDS expire under WHMIS?
No, but they must always reflect current hazard information. Regular reviews are highly recommended. - Who ensures SDS are current in the workplace?
Suppliers are responsible for issuing updates. Employers must maintain current versions and make them accessible to workers. - What triggers require SDS changes?
New health data, product reformulations, or regulatory changes that affect classification or safety guidance. - Can SDS be stored digitally?
Yes, as long as they are accessible to employees without delay and are backed up.